Why does the human body not store oxygen like fat and water?

The human body or our maker, in their wisdom, decided to store water and fat in the body but not oxygen.
Why, you ask? Well, it has its reasons, and they might just make you appreciate this peculiar design choice:
- Oxygen is a party animal: It's required non-stop for cellular respiration, where our cells make energy to keep us alive and kicking. Oxygen loves being the life of the party, so it keeps moving from one cell to another without taking a break. There's no time to sit idly in storage!
- Oxygen rides with the cool kids: Hemoglobin, the protein in red blood cells, plays chauffeur to oxygen molecules. Like a VIP shuttle service, hemoglobin picks up oxygen from the lungs and drops it off at the trendiest cellular hotspots. Who needs storage when you have such a fancy transport system?
- Oxygen is too reactive for its own good: It loves mingling with other elements and forming compounds. Storing large amounts of oxygen would be like hosting a chemistry party that gets out of control, producing harmful reactive oxygen species (ROS) and causing cellular mayhem. Nobody wants that kind of drama!
- Oxygen's got a mini-storage unit: While it doesn't have a massive storage space, oxygen does keep a small stash in myoglobin, a protein found in muscles. It's like a tiny walk-in closet for oxygen to hang out in during high-demand situations, such as intense exercise. However, this storage is limited, and oxygen prefers to be out and about anyway!
Now we know. The human body doesn't store oxygen like it does water and fat because it's too busy partying with our cells, hitching rides with hemoglobin, trying not to cause chemical chaos, and occasionally chilling in its small myoglobin storage. Our respiratory system is perfectly happy to keep the oxygen supply flowing by constantly breathing in fresh oxygen from the environment.
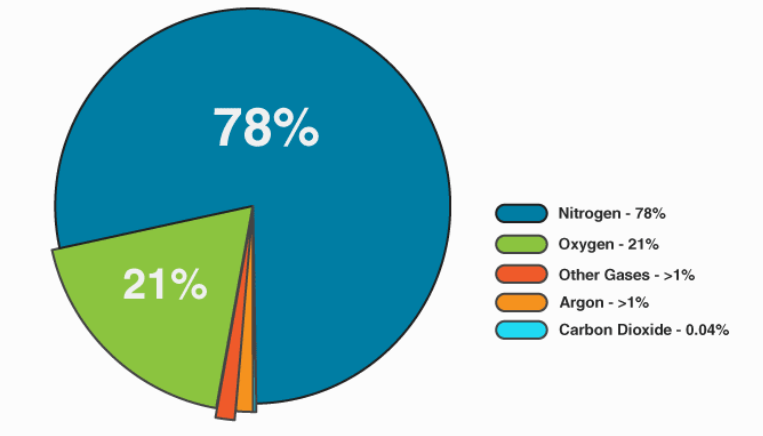
BTW: we don't breathe pure oxygen. We breathe air which has other gases as well and oxygen constitutes about 21% of that mixture.
Composition of air we inhale:
Composition of air we exhale:
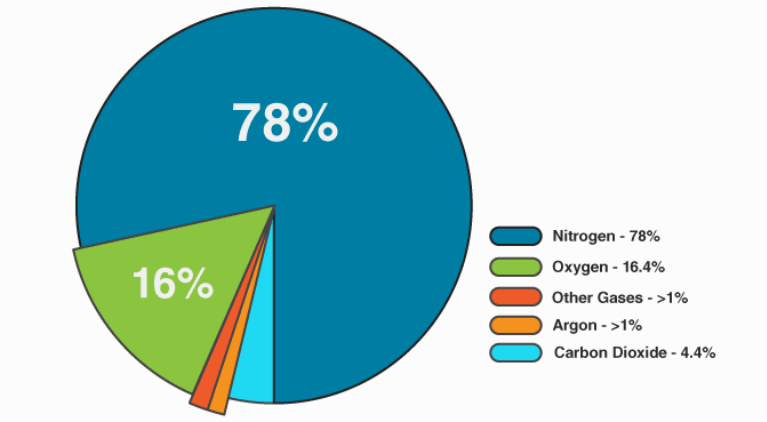
The respiratory process filters out other gases and consumes the oxygen our cells need to operate.
Breathing pure oxygen at atmospheric pressure is toxic. We can't survive that for more than a week.
Oxygen toxicity depends, not on oxygen concentration but on partial pressure.
Partial pressure is calculated by ambient pressure multiplied by concentration. At atmospheric pressure this is 21kPa (considering 21% oxygen content in the air).
Breathing 100% pure oxygen at atmospheric pressure gives a partial pressure of 100kPa, which is toxic. And, a partial pressure of 400kPa (in a hyperbaric chamber, for instance) leads to seizures.
Breathing pure oxygen at one-fifth of atmospheric pressure gives a partial pressure of 20kPa, which is safe to breathe for indefinite periods. This is the concept behind pumping pure oxygen into space shuttles or astronaut suits because of the reduced atmospheric pressure.
Uncontrolled decompression is another challenge. More on that some other day.



